COLOTECT, DNA Screening Test for Colorectal Cancer
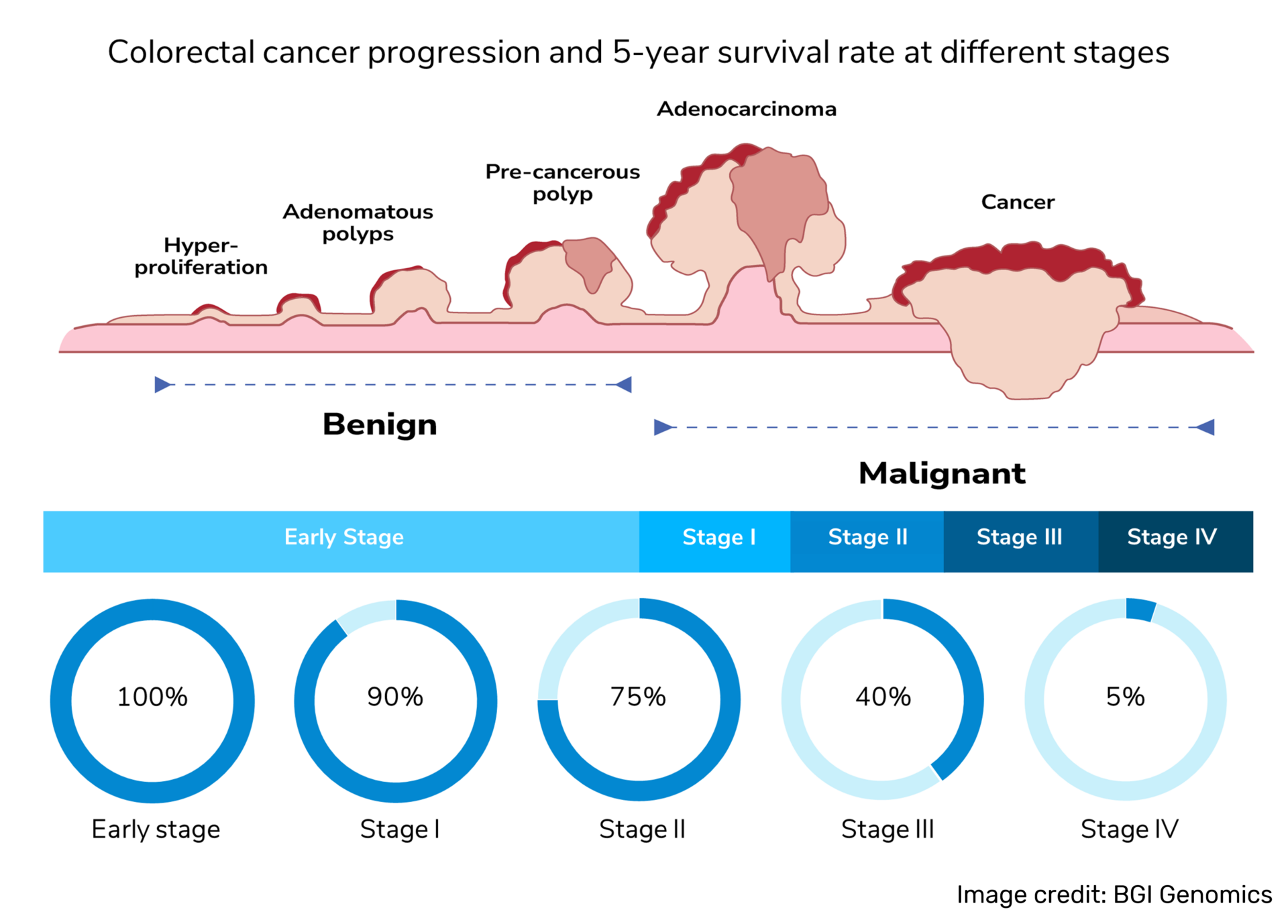
Colorectal cancer diagnosis at late-stage could result in: patient has to undergo a more complicated treatment course, lower chance of recovery, decreasing the quality of life and higher financial burden incurred from long-term treatment and regular follow-up. Thus, it is crucial for the early diagnosis of colorectal cancer.
There are many options for colorectal cancer screening, and some are designed to detect common colorectal cancer symptoms such as occult blood. However, these tests can miss to detect colorectal cancer if the patient does not present with symptoms during early stage of the disease and limit early cancer diagnosis and treatment.
COLOTECT is an easy-to-do test as the procedure is painless, performable at home or clinic and does not require any bowel preparation or sedation which can be a barrier to colonoscopy. Non-invasive faecal DNA testing can also be used as a screening tool for individuals who are at average risk of developing colorectal cancer. Individuals aged 50-74 who have no symptoms or individual with family history of colorectal cancer are recommended to go for COLOTECT screening, and the test can be repeated for every 3 years. Not only frequent colorectal cancer screening helps saving life, but also assure better life quality and more cost-effective in terms of colorectal cancer management7.

References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I et al. 2021 Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 71(3), 209-249.
- Azizah A, Hashimah B, Nirmal K, et al. Malaysia National Cancer Registry Report 2012-2016. National Cancer Institute Ministry of Health Malaysia; 2019. Report No. MOH/P/IKN/05.19 (AR).
- Kim JH & Park SC. 2018 Syndecan-2 methylation as a new biomarker for early detection of colorectal neoplasm. Gut and Liver, 12(5), 479.
- Hu YH, Ma S, Zhang XN, Zhang ZY, Zhu HF, Ji Y et al. 2019 Hypermethylation of ADHFE1 promotes the proliferation of colorectal cancer cell via modulating cell cycle progression. OncoTargets and therapy, 12, 8105.
- Li HH, Cai X, Shouse GP, Piluso LG & Liu X. 2007 A specific PP2A regulatory subunit, B56γ, mediates DNA damage‐induced dephosphorylation of p53 at Thr55. The EMBO journal, 26(2), 402-411.
- Fang Y, Peng J, Li Z, Jiang R, Lin Y, Shi Y et al. 2022 Identification of Muti-Omic Biomarkers from Fecal DNA for Improved Detection of Colorectal Cancer and Precancerous Lesions. medRxiv, 2022-11.
- Peng J, Zhu S, Wang Y, Gao Y et al. 2022 Clinical validation of a novel stool DNA-based test in a large scale, prospective, real-world CRC screening study.
- Yang Z, Shi M, Liu M, Wang Z, Huang H, Wang S et al. 2022 A cost-effectiveness of Fecal DNA methylation test for colorectal cancer screening in Saudi Arabia. medRxiv, 2022-11.
